INTRODUCTION
Metoclopramide [(MCP), Reglan®, Clopra®, Gimoti® etc.] known as (4-amino-5-chloro-[N]-[2-(diethylamino) ethyl]-2-methoxybenzamide) (Figure 1) is primarily used as an antiemetic or a gastrointestinal prokinetic drug in adult and children medicine as well as for gastroparesis in patients with diabetes nausea, vomiting, a feeling of fullness satiety, and loss of zest.1,2,3,4
Because of having a wide application and a great therapeutic in empirical and clinical medicine, much study have concentrated on its determination in dosage forms. For the quantification of MCP in pharmaceutical products and biological fluids, several analytical methods have proposed such as high performance liquid chromatography (HPLC),5,6 spectrofluorimetric,7 electrochemical,8,9,10 chemiluminescence,11 tandem mass spectrophotometry.12 Liquid chromatography is the official method for assay of MCP in the British Pharmacopoeia and United States Pharmacopoeia.13,14 Several of these mentioned procedures are not simple for routine analysis, costly or complicated instruments, may require heating, or have relatively poor selectivity.
Titrimetry and visible spectrophotometry are perhaps the most widely techniques reported for the determination of MCP in pharmaceuticals.15,16,17,18,19,20,21,22 Further, literature surveys revealed the use of spectrophometric method for estimation of MCP in the injection dosage form by direct ultraviolet (UV) spectroscopy at a wavelength of 270 nm with maximum absorbance using 0.1 M HCl as solvent.23 Injections have little or no interference compared to other dosage forms such as tablets or suspensions since they contain almost no excipients. A stability indicating method has been developed for quantification of MCP in bulk by UV spectrophotometry in presence of its degradation products. The limit of detection (LOD) and limit of quantification (LOQ) values were found to be 3.26 µg/mL and 9.89 µg/mL, respectively.24
The redox titrimetric method of analysis is a possible alternative the various available analytical methods they are not only sensitive, precise, cost-effective; but relatively accurate.
This investigation develops simple, sensitive, and cost-effective methods for the determination of MCP in pure preparation, injection, and tablets using redox techniques.
The titrimetric method was based on the N-oxidation reaction involving the use of potassium hydrogen peroxymonosulfate as the titrant. A known excess of reagent is added and, after a specified time, the residual reagent is determined iodometrically. The spectrophotometric method depends upon the oxidation of MCP with Oxone® in alkali medium (pH: 9.9) followed by coupling with iodide in acidic medium (pH: 4.0) to give a yellow-brown colored chromogen (triiodide) with a wavelength of maximum absorption at 350 nm.
MATERIALS AND METHODS
Reagents
MCP hydrochloride, 98.8% ACROS OrganicsTM; CAS 7232-21-5, C14H23Cl2N3O2; melting point 171-173°C.
MCP was oxidized to a MCP hydrochloride N-oxide with the aid of potassium peroxymonosulfate (KHSO5), a component in the commercial product called Oxone®; formula of Oxone®: 2KHSO5 ·KHSO4 ·K2SO4; CAS number. 70693-62-8, extra pure, min 4.5% active oxygen, ACROS Organics™; its formula weight is 614.78 g/mol. Moreover, it is considered “green”-oxidizing agent because of its nontoxic effects.
Standard drug solution
A stock standard solution of pure preparation containing 1 × 10-2 mol/L (3.363 mg/mL) MCP was prepared in double-distilled water and used in titrimetric method.
Injection: 2 mL «Polpharma» (Poland) N 5 injection containing active substance of MCP hydrochloride 10 mg;
Excipients: Sodium pyrosulfite 2 mg, sodium chloride 14 mg, water for injection up to 2 mL; and tablet containing MCP.
Tablet: Cerucal® 10 mg N50, AWD Pharma, manufactured «PLIVA Hrvatska» (Croatia). Each tablet contained active substance MCP hydrochloride monohydrate 10.54 mg (which corresponds to anhydrous MCP hydrochloride 10.00 mg);
Excipients: Potato starch 36.75 mg, lactose monohydrate 76.65 mg, gelatin 2.16 mg, silicon dioxide 2.60 mg, magnesium stearate 1.30 mg. According to the quality certificate, the quantitative content of MCP hydrochloride (C14H23Cl2N3O2) was 9.9 mg. They were purchased from local commercial sources.
Apparatus: Unicam SP 800 instrument, Beckman DB spectrophotometer. 10 mL microburette. Air thermostat TS-80 m.
Solutions: KHSO5, 1.73 x 10-2 mol/L from analytical-grade Oxone. Potassium iodide, 5% analytical-grade potassium iodide. Iodide, 1 mol/L from analytical-grade potassium iodide. Sulfuric acid, c(H2SO4)= 0.5 mol/L, volumetric solution. Sodium thiosulfate standard solution [c(Na2S2O3·5H2O)= 0.1 mol/L].
Buffer solutions: 20 g/L of potassium hydrogen phthalate (pH: 4.0); 0.2 M solution potassium pyrophosphate with values of 8.6 and 9.3. For pH: 9.9: dissolve 28.62 g of sodium carbonate (Na2CO3 · 10H2O) and 8.40 g of sodium bicarbonate (NaHCO3) in 1 L volume-distilled water.
Synthesis of MCP N-oxide
A mixture of MCP hydrochloride (0.71 g, 2 mmol), (0.76 g, 2.5 mmol) Oxone® (2KHSO5·KHSO4·K2SO4) and 20% aqueous solution of sodium carbonate (25 mL) and solution was stirred at room temperature until disappearance of the starting material. The solution was treated in an ultrasonic bath for 15 min. Water was removed from the mixture by evaporation under vacuum and the resulting solution was lyophilized at room temperature. The residue was taken up with ethyl acetate to give MCP N-oxide in quantitative yield as a colorless solid. Further information about maximization of yield is already published.25
Titrimetric assay
The procedure for quantitative determination of MCP in pure preparation
MCP (0.35404 g) dissolved in 100 mL of double distilled water. Using a pipette, volumes (10 mL) of a prepared solution were accurately transferred to 100 mL measuring flask, 10 mL of 0.02 mol/L previously prepared solution of the KHSO5, pH: 9.9 buffer solution (75 mL) and water were added to make the final volume of a 100 mL solution. They were mixed to homogeneity (start stop clock). Within a chosen period (10-15 min), 20.00 mL was pipetted to the reaction mixture in a 150 mL conical flask. 4.0 mL of 0.5 mol/L sulfuric acid and 5 mL of 5% solution of potassium iodide were added while shaking. The formed iodine was titrated with 0.01 mol/L sodium thiosulfate using a micro buret until the mixture turned colorless. The blank titration was repeated, omitting MCP (control titration).
The MCP content in the pure preparation, % (X), was calculated using the following equation:
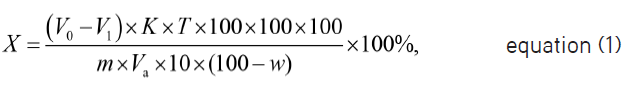
where V0 is 0.01 mol/L sodium thiosulfate volume used for titration in blank determination, mL;
V1 is 0.01 mol/L sodium thiosulfate volume used for titration in procedure, mL;
K is correction factor of concentration of concentration 0.0100 mol/L sodium thiosulfate solution concentration;
T is a mass of a substance, which reacts with 1 mL of 0.01 mol/L sodium thiosulfate, g/mL;
100 is volumetric flask capacity, mL;
Va is volume of reaction mixture taken for analysis, mL;
m is mass of a substance to be determined, g;
w is substance moisture content, %;
10 is volume of the pipette, which is used for measuring the solution aliquot, mL.
1.00 mL of standard 0.0100 mol/L sodium thiosulfate solution is equivalent to 0.0016813 g/mL of MCP, which should be 99-101% in the preparation terms of the anhydrous base.
Quantitative determination of MCP in tablets Cerucal® 10 mg
Twenty tablets containing MCP were weighed and ground into fine powder for methods A and B, and a weighed quantity of the crushed tablet equivalent to 200 mg MCP (2.5880 g) was transferred to a 100 mL flask and shaken with 60 mL water for about 20 min, then made up to the mark with water, mixed and filtered using a Whatman N42 filter paper. Transfer was carried using a pipette to accurately measure the volume (15 mL) of the prepared solution to 100 mL measuring flask. Same procedure as mentioned in the procedure for determination of MCP in pure preparation was repeated.
MCP content in one tablet, mg (X), has been calculated by the following equation:
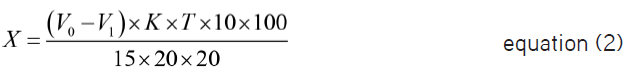
where V0 is 0.01 mol/L sodium thiosulfate volume used for titration in blank determination, mL;
V1 is 0.01 mol/L sodium thiosulfate volume used for titration, mL;
K is the correction factor of concentration of concentration 0.0100 mol/L sodium thiosulfate solution concentration;
T is a mass of a substance, which reacts with 1 mL of 0.01 mol/L sodium thiosulfate, g/mL (1.6813 mg/mL MCP);
15 is the volume of a dosage form solution taken for analysis, mL; 20 is volume of the pipette, which is used for measuring the solution aliquot, mL; 100 is volume of flask used, mL; 20 is number of tablets taken for analysis.
1.00 mL of standard 0.0100 mol/L sodium thiosulfate solution corresponds to 0.0016813 g/mL of MCP, which should be 95-105% in the preparation in terms of the anhydrous base.26
Quantitative determination of MCP in 0.5% injection dosage form
Accurately measured volumes (10.0 mL) of solution for injection (content five-six ampoules) were transferred using a pipette to a 100 mL measuring flask and same procedure as for the determination of MCP in pure preparation was repeated. The titration was repeated without the addition of a buffer solution (pH: 9.9), the same volume of double distilled water was used in its place. For 0.1 mol/L hydrochloric acid solution, 2 mL instead of 10 mL was added (control titration).
MCP content in the solution for injection, g to 100 mL (X), was calculated using the following equation:
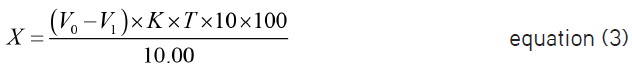
where V0 is 0.01 mol/L sodium thiosulfate volume used for titration in blank determination, mL;
V1 is 0.01 mol/L sodium thiosulfate volume used for titration in procedure, mL;
K is the correction factor of concentration of concentration 0.0100 mol/L sodium thiosulfate solution concentration;
T is a mass of a substance, which reacts with 1 mL of 0.01 mol/L sodium thiosulfate, g/mL;
10.00 is volume of a dosage form solution taken for analysis, mL;
100 is recalculation to 100 mL;
10 is dilution factor.
1.00 mL of standard 0.0100 mol/L sodium thiosulfate solution corresponds to 0.0016813 g/mL of MCP, which should be 90-110% in the preparation in terms of the anhydrous base.
Spectrophotometric assay
Procedure for obtaining results for calibration graph
Into 50 mL flask, an aqueous solution (standard) of MCP (0.5-5.0 mL) was added, and then 1.73 x 10-4 mol/L KHSO5 solution (3.00 mL). The flask was washed with adequate water to increase the volume to 8.0 mL, to which the buffer solution (20 mL) was added and maintained for 10-15 min. Then 0.5 mol/L sulfuric acid solution (1 mL) was added and the volume adjusted to 45 mL with the solution containing 20 g/L of potassium hydrogen phthalate; 5 mL of 5% solution of potassium iodide was also added (5 mL). Prepared solutions were maintained for 60 s and the absorbance at 350 nm was measured against distilled water. A control measurement was carried out similar to the working experiment, with the difference that double-distilled water was used instead of the investigated drug solution. The difference in optical densities obtained in the control and working experiments, respectively (ΔA), was plotted versus the concentration of MCP.
Spectrophotometric determination of MCP in tablets Cerucal® 10 mg
Twenty tablets were weighed and pulverized. The equivalent to 200 mg MCP was dissolved in double distilled water and filtered; the residue was rinsed, volume adjusted to 100 mL, and further diluted with same diluent to obtain the working concentration (1 x 10-4 mol/L). The prepared aqueous solution of MCP (2.00 mL) and 1.7 x 10-4 mol/L KHSO5 solution (3.00 mL) were pipetted into a 50 mL graduated flask, and subsequent addition of reactants, diluents and buffers as in the above-written spectrophotometric procedure for obtaining results for calibration graph. The prepared solution was made up to 50 mL (after being kept for 60 seconds) and measured absorbance measured; the amount of MCP present in the sample was computed from the calibration curve.
Spectrophotometric determination of MCP in 0.5% injection dosage form
Accurately measured volumes (5.0 mL) of solution for injection were transferred using a pipette to a 100 mL measuring flask and brought to a final volume of 100 mL with water. Then, an aqueous solution of MCP (3.00 mL) was transferred to a 50 mL graduated flask and same procedure as further written in spectrophotometric determination of MCP in tablets Cerucal® 10 mg was carried out. For control measurement: In place of a sulfuric acid solution and a buffer solution with a pH of 9.9, double-distilled water was used.
Recovery studies
The recovery was calculated as the percentage of values obtained using standard pharmacopeial method as provided by the certificate of analysis from quality control laboratory.
RESULTS AND DISCUSSION
The stoichiometry of the reaction for synthesis of MCP N-oxide was assessed and found to be 1:1 (MCP: KHSO5). The study of kinetics showed that the optimal time of quantitative interaction is 10-15 min at pH: 9.9 (Figure 2).
The interaction of MCP with potassium hydroperoxy monosulfate can be represented by the scheme in Figure 3. During study of the influence of iodide concentration on the absorbance of the final solution, steady absorbance was obtained when the iodide concentration was 0.1 mol/L. The molar absorptivity at 350 nm was found to be 2.46 x 104 (Table 1, Figure 4). Calculations based on the association constant for tri-iodide (logK: 2.9) show that more than 97% of the iodine was found as tri-iodide in solutions containing >0.05 mol/L iodide; thus, a percentage of the reduction in molar absorptivity observed in lower iodide concentrations may be explained due to incomplete formation of tri-iodide. Notwithstanding, an explanation for the occurrence of such significant reduction in the presence of higher KHSO5 concentrations may currently be difficult; as Nisli and Townshend27 suggest, it may be due to incomplete KHSO5-iodide reaction under these conditions.
These conditions were the basis for developing a new-oxidimetric method for the quantitative determination of MCP using potassium hydrogen peroxymonosulfate as an analytical reagent. In titrimetric method, when the reaction between the tertiary amine group present in MCP with KHSO5 was completed, the excess KHSO5 was detected iodometrically.
The results of MCP determination in pure substance by oxidimetry-using potassium hydrogen peroxymonosulfate (Oxone®) are shown in Table 2.
The results for both titrimetric and spectrophotometric determination of MCP in tablets and solution for injection dosage forms are presented in Table 2. Results of recovery studies show that in all cases that δ* was within the recommended limits. In spectrophotometric determination of MCP, LOQ (10S) is 4.61 x 10-7 mol/L.
CONCLUSION
Two simple methods for the determination of MCP in tablets and in injection were developed. These methods are based on N-oxidation reactions. This spectrophotometric method is the simplest method ever reported and the iodometric titration method is the first ever reported for the determination of MCP. The titrimetric method is applicable over wide linear dynamic ranges and was successfully applied to the tablets and injection. The statistical characteristics and recovery study information revealed the reproducibility and accuracy of the methods. Besides the simplicity and sensitivity of the procedures, the relative cheap cost of apparatus and reagents is also an advantage. The methods are also useful due to high tolerance limit for common excipients found in pharmaceutical formulations. These merits coupled with the use of simple and relatively inexpensive instruments and high selectivity of the methods suggest their possible application in routine quality control laboratories.
Ethics
Ethics Committee Approval: Not applicable.
Informed Consent: No part of this work was experimented on human or animals.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Concept: M.B., Design: D.A.U., Data Collection or Processing: M.B., O.O.M., V.P.M., Analysis or Interpretation: M.B., D.A.U., O.O.M., V.P.M., Literature Search: D.A.U., O.O.M., Writing: M.B., D.A.U.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.