INTRODUCTION
A novel drug delivery system that delivers drugs at a preset rate set as per the need, pharmacologic aspects, drug profile, physiological conditions of the body, etc. In current times, no single drug delivery system meets all the standards; however, efforts are created through novel approaches.
The aim of novel drug delivery systems is targeted and controlled drug delivery. Colloidal delivery and nanotechnology have attracted the most interest because of promising systems for having a localized result.1
The delivery of drugs using colloidal particulate carriers such as proniosomes is dry and free-flowing preparation coated with a surfactant. To form a multi-lamellar noisome, proniosomes are rehydrated directly within minutes by transient agitation. Niosome suspension is appropriate for giving medication by different routes. They are promising candidates for industrial applications as they can transport, distribute, store, and process easily. Therefore, proniosomes can be another alternative to liposomal and other vesicular drug delivery systems for the entrapment of both polar and non-polar medications.2
Proniosomes improve effectivity, scale back or eliminate adverse effects and enhance therapeutic actions of medicine. They are accustomed to avoid the gastrointestinal tract incompatibility, pre-systemic metabolism, and unwanted adverse effects related to oral delivery. Additionally, they maintain therapeutic levels of drug for an extended time, decrease the frequency of administration and improve patient compliance.3,4,5
This article in brief reviews the types, fabrication, characterization, and pharmaceutical applications of proniosomes.
Structures of proniosomes
Proniosomes are microscopic lamellar structures, hexangular structures, and blackish structures, where their location is clear, semi-transparent, and semi-solid gel-like structures (Figure 1). Consistent with their methodology of preparation, proniosomes are unilamellar or multi-lamellar. They even have bilayer in their structure having hydrophilic ends that are exposed on the surface and hydrophobic chains that face one another within the bilayer inside the vesicles. Bilayer consists of non-ionic surface-active agents. To create a bilayer surfactant molecule, offers direction in such a way that hydrophilic ends of the non-ionic surfactant are arranged toward the outside, whereas the hydrophobic ends exist in the opposite direction. Hydrophilic drugs are placed at intervals in the area encircled within the vesicle and the hydrophobic medication is implanted within the bilayer. For association, in liquid media, proniosomes attach to cholesterol with different categories of non-ionic surfactant like alkyl radical or dialkyl polyglycerol ether.6
Materials used for the preparation of proniosomes
Surfactant: Surfactants, especially non-ionic surfactants are the key structural components in the preparation of proniosomes. These surfactants do not have any charge as they possess a polar head and non-polar tail. So, their stability, toxicity and compatibility is higher than other surfactants. The non-ionic surfactants have wet and emulsifying effects by which they improve the solubility and permeability of drugs. The hydrophilic-lipophilic balance (HLB) value is critical for selecting surfactants and HLB value between 4 and 8 is compatible with vesicle formation by proniosomes. It is difficult for hydrophilic surfactants to achieve a high concentration because of the high liquid solubility of hydrophilic surfactants. Therefore, aggregation and conglutination to form a proniosomal lamellar structure would be absent (Table 1).7
Cholesterol: Cholesterol can interact with non-ionic surfactants and regulates the physical and structural properties of proniosomes.8 It improves the stability and rigidity of the proniosomal membrane and controls drug permeation through the membrane. Depending on the HLB value of the surfactants, the amount of cholesterol required for the preparation of proniosomes is determined. When the HLB value is above 10, the amount of cholesterol to be increased to cover the larger groups.9 But entrapment efficiency (EE) of the prepared formulation is decreased10 above a certain level of cholesterol, possibly due to a decrease in volume diameter.11
Lecithin: Lecithin is a phospholipid that acts as a membrane stabilizer in the formulation of proniosomes. The most common lecithins that are used in the formulation are soya and egg lecithin and it has been reported that hydrogenated-type lecithins have advantages over not hydrogenated lecithins, give increased rigidity of the cholesterol and help in the formation of tight vesicles.12 Double bonds in non-hydrogenated lecithin allow the molecular chains to bend (conformational rotation), which prevents tight contact with the adjacent molecules on forming the niosomal membrane. This results in low rigidity and high permeability of the membrane.
Hydration medium: Generally, the hydration medium used in proniosomes is phosphate buffer. Depending on the solubility of the encapsulated drug, the pH of the buffer is selected.13 Ruckmani and Sankar14 ascertained that drug leakage increased with the increase in the volume of hydration medium but simultaneously, EE increases, when the hydration time was increased from 20 to 45 min.
Organic solvent: The solvent can act as a penetration enhancer. It also greatly affects the size of the vesicles formed. The size of the vesicle and permeation rate of the drug in a proniosomal formulation are influenced by the type of alcohol. Different sized vesicles are formed using different alcohols as they have the order: >> isopropanol < butanol < propanol < ethanol.15
Carrier material: Carrier materials accommodate the drug in the proniosomal formulations. Carriers should have safe, non-toxicity, free-flowing properties. They should possess low solubility in the solution of loaded, but good solubility in water for ease of hydration. They increase the surface area and impart flexibility to the proniosomes. The frequently used carrier materials are sorbitol, mannitol, maltodextrin, glucose monohydrate, spray-dried lactose, sucrose stearate, and lactose monohydrate.16
Preparation method of proniosomes
A drug that has poor aqueous solubility, low bioavailability and dissolution, poor membrane permeability, low absorption profile, excessive metabolism, variable plasma concentration, and poor patient efficiency is suitable to encapsulate into proniosomes.17 Three methods are available for proniosomal drug formulation (Figure 2).
Slurry method
In this method, a single or a mixture of organic solvent is used in the preparation of a stock solution of surfactant and membrane stabilizer. The drug and carrier are dissolved in a membrane stabilizer solution and all the components are mixed until a slurry is formed. With the help of a rotary evaporator at specified conditions (e.g. 50-60 rpm, 45 ± 2°C temperature, and 600 mm of Hg pressure), the slurry is dried, and the free-flowing product is obtained. The obtained free-flowing dried material is further dried with the help of a desiccator at room temperature under vacuum to get proniosomes.18
Slow spray coating method
The slow spray-coating method is carried out by spraying organic solution, surfactant, cholesterol, and drug onto the carrier and then removing the solvent using a rotary evaporator under controlled conditions at 65-70°C for 15-20 min. Until the desired surfactant loading has been achieved, the process is continued and repeated. The vaporization should be carried on until the powder becomes completely dry.19,20
Coacervation phase separation method
Most of the proniosomal gel (PNG) is prepared by this method. In this method, exactly measured amounts of drugs, surfactants, and cholesterol are placed in a clean and dry glass vial having a wide opening. Then, the solvent is added and warmed in a water bath at 60-70°C until the surfactant and cholesterol is fully dissolved. To prevent the evaporation of the solvent, the open end of the vial should be covered with a lid. Followed by the addition of an aqueous phase in the vial, the mixture was warmed in the water bath to get a clear solution. It is then cooled at room temperature, between this time PNG is produced from the dispersion.21
Evaluation of proniosomes
A group of properties of proniosomes can be evaluated using different methods. These methods are described below.
Vesicle size and shape
On hydration, proniosomes are converted to globular-shaped niosomes. The size and morphology of the niosomes can be determined by optical microscopy, scanning electron microscopy (SEM), photon correlation microscopy, transmission electron microscopy (TEM), and freeze-fracture electron microscopy.22 Dynamic light scattering (DLS) method is applied to measure the vesicular size distribution. DLS essentially measure fluctuations in scattered light intensity due to diffusing particles, the diffusion coefficient of the particles can be determined. The vesicle size of the noisome is measured without agitation and with agitation. If hydration is performed without agitation, biggest size is formed.23
Angle of repose measurement
The angle of repose of dried proniosomes prepared using the slurry and spray coating method is measured by the funnel and cylinder technique.
Zeta potential (ZP)
The stability of the particle can be ensured with the value of ZP. This is ascribed to the electrostatic repulsion between particles with the same electric charge that causes the segregation of the particles. A high ZP value leads to increased repulsive interactions in charged particles and prevents the agglomerate formation between the particles. This ensured uniform size distribution in proniosomes. A proniosomal formulation having ZP value minimum ± 30 mV is considered a physically stable formulation. So, aggregation of particles can be avoided.24,25
Osmotic shock
An osmotic shock study helps in the determination of vesicle size changes. For this, the proniosomal formulations are incubated in different types of solution like hypertonic, isotonic, and hypotonic solutions for 3 h. Changes in vesicle size are detected by an optical microscope.23
Entrapment efficiency (EE)
To study EE, we should separate the free drug using several techniques like dialysis, gel filtration, ultracentrifugation, column chromatography, and freeze-thawing. Two techniques can be applied to measure EE. One is the destruction of the proniosomal vesicle with propane (50%) or triton (0.1%) and the entrapped drug is determined. Another method is that after the destruction of the vesicle, the un-entrapped drug is measured.26
The percentage of entrapment was calculated using the following formula:
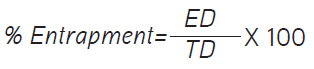
where ED is the amount of entrapped drug and TD is the initial amount of drug.
Drug content
The calibration curve is used to calculate drug content. For this, proniosomes are lysed with methanol in a volumetric flask by shaking for 15 min. Then, the stock solution is prepared with methanol. With the help of phosphate buffer, 10% solution is prepared from the stock solution. Aliquots are withdrawn and absorbance is measured followed by a drawing of calibration curve.24
Particle size (PS) measurement
One of the important criteria for prepared proniosomes is PS. With the help of SEM, the uniformity of PS and surface characteristics is measured. Optical microscopy is used to confirm the formed vesicles after hydration of proniosome.26
Rate of spontaneity
The rate of spontaneity is the measure of the number of niosomes formed following hydration of proniosomes. To determine the rate of spontaneity, PNG is transferred and spread uniformly along the walls of the small stoppered glass tube container. Then, NaCl (0.154 M) was added with caution and placed to one side without any turbulence. With the help of Neubauer’s chamber, the number of niosomes eluted from proniosomes is calculated.27
In vitro dynamics study
The in vitro release study is a critical test to assess the safety, efficacy, and quality of nanoparticle-based drug delivery systems. In vitro, drug release kinetics of the prepared proniosomes can be determined using a Franz diffusion cell, Keshary-Chien diffusion cell, dialysis membrane, reverse dialysis, and United States Pharmacopeia dissolution apparatus type 1.28
Stability study
To ensure the stability of the prepared proniosomes, they are placed at a various temperatures, freezing temperature (2-8°C), normal temperature (25 ± 0.5°C) and elevated temperature (45 ± 0.5°C) for 1-3 months and the change in drug content and mean vesicle diameter is observed at a different time interval. The International Conference on harmonization (ICH) guidelines propose dry proniosomes powder should be studied for the accelerated stability at 75% relative humidity and 40°C as per international geographical zones and geographical conditions.29
Proniosomal drug delivery through different routes
Oral routes
The oral route of drug administration is the most preferred route for drug delivery. But bioavailability of the orally administered drug is sometimes affected by first-pass metabolism, instability in the gastric environment, low permeability through the intestinal epithelium. In some cases, absorption of the drug may be altered due to the presence of food. Thus, to improve the bioavailability of the oral drug, different nanocarriers are engaged. Oral proniosomes are one them that can solve the limitations of the conventional oral dosage form.30 In vitro release kinetics of oral vinpocetine (VP) prepared using the slurry method indicated a faster release rate of reconstituted niosomes in contrast to VP suspension at pH 6.8 or 7.2 phosphate-buffered saline. In vivo pharmacokinetic study data also showed a better correlation with the in vitro data.31 Oral acemetacin also prepared using the slurry method in proniosomal powder and tablet formulations, displayed better pharmacokinetic properties.32 Lornoxicam is a widely used analgesic drug that belongs to the non-steroidal anti-inflammatory group. Proniosomal form of lornoxicam showed significantly higher (p<0.05) transmucosal flux across the oral mucosa than lornoxicam containing carbopol gel and the diffusion of lornoxicam was higher (more than two folds) in proniosomal formulation.33 Proniosomal telmisartan tablets prepared with surfactants having different HLB values (span 40 and brij 35), cholesterol (20-50%), and phospholipids (egg yolk and soybean). In vitro as well in vivo comparative study showed extended drug release with a higher Cmax. The Cmax was increased 1.5 fold while AUC0-∞ also increased significantly 3 fold compared with the commercial tablet. The sustained release pattern of telmisartan was indicated by tmax, which was increased 3 fold in contrast to conventional tablets. The relative bioavailability was also increased by 3.2 fold (Table 2).34
Ocular routes
Proniosomes are one of the promising methods in ocular drug delivery. In this route, PNGs in the ocular route provide several advantages like extended and sustained action, enhanced corneal residence time, prevented enzymatic degradation of drugs in tears, and ultimately improve ocular bioavailability.35 Lomefloxacin HCl prepared using the coacervation phase separation method. The results showed that the area under the curve (AUC) of proniosomal formulation is higher (722.45 ± 0.01) than the marketed product (126.25 ± 0.049).36 Li et al.37 developed proniosomal formulation of tacrolimus, an immunosuppressive agent for topical ophthalmic delivery containing lecithin and poloxamer 188 as surfactants, cholesterol. In vitro studies in rabbit cornea of stable tacrolimus-loaded proniosomes showed enhanced precorneal permeation and retention of tacrolimus. The in vivo ocular irritation test of 0.1% tacrolimus-loaded proniosomes in rat eyes for 21 successive days (four times in a day) showed no irritation and good compatibility with the cornea. Proniosomes were also found to prolong the survival of corneal grafts and showed practical corneal anti-allograft rejection efficacy in the xenotransplantation model. Curcumin-loaded PNG was developed for treating ocular inflammation with the help of cremophore RH, lecithin, and cholesterol. Selected PNG showed 3.22 fold and 1.76 fold higher permeability rates than curcumin dispersion and its freeze-dried form, respectively.38 This formulation could be an effective, better biofriendly alternative for the therapy of inflaming eyes.
Emad Eldeeb et al.39 studied on brimonidine tartrate (BRT) proniosomal formulation used in glaucoma developed using the coacervation phase separation method. They used two surfactants having higher and lower HLB values, namely tween 80 (HLB 15) and span 60 (HLB 4.7), respectively. The results of BRT PNG showed a 7.90 fold increase in mean residence time compared to the marketed product.
Simultaneously, the relative area under the plasma concentration-time curve over the last 24 h dosing interval (AUC0-24) value was also increased to 5.024 folds. Draize test also ensured that the formulations had no ocular irritation.39 Dorzolamide-HCl (Dorz) is an anti-glaucoma drug soluble in water. The PNG of this drug was developed to sustain its effect and to reduce dosing frequency. Here, the gel was developed by using L-α-lecithin, cholesterol, span 40 using the coacervation phase separation method. In vivo results showed a maximum reduction in intraocular pressure (IOP) of 32.6 ± 2.7 at 1.5 h. On the contrary, the PNG showed a sustained decrease in IOP with a maximum value of 45.4 ± 8.2 at 6 h, which was significantly higher than standard formulation. Even after completion of the experimental period (8 h), the % dec in IOP was 19.5 ± 9.2. This implies a prolonged release of Dorz PNG from the optimized formulation.40
Pulmonary routes
With the aid of the pulmonary route, one can easily treat respiratory diseases than other delivery methods. Through this route, drugs can be directly applied within the lungs. Drug-loaded particles like liposomes dispensed through aerosol can easily distribute to the bronchi and lungs and prolong the release of the drug. Liposomal delivery also has minimum systemic side effects due to localized action to the lungs. But liposomes may be degraded by oxidation or hydrolysis. So, the proniosome can be an option to overcome the limitations of the liposome.41
For pulmonary drug delivery, the air-jet nebulizer is known very well. Proniosome-derived niosomes of cromolyn sodium were prepared by Abd-Elbary et al.26 They used sucrose stearates in the formulation. The results exhibited a controlled release of drugs from the proniosome-derived niosomes compared to standard drug solution. Furthermore, high nebulization efficiency and physical stability were also achieved.26
Likewise, aerosol properties of beclometasone dipropionate (BDP) niosomes using Aeroneb Pro and Omron Micro Air vibrating mesh nebulizers and Pari LC Sprint air-jet nebulizer were investigated by Elhissi et al.42 The study demonstrated that the satisfactory EE of BDP in proniosome-derived niosomes and the value was higher than that in conventional thin film-made niosomes.42
Vaginal routes
Vaginal drug delivery is one of the favorable routes to target the disease associated with female health issues. It offers both the local and systemic delivery of drugs. Usually, different categories of drugs like antibiotics, antifungal, antiprotozoal, antiviral, labor-inducing agents, spermicidal agents, steroids, etc. are delivered through the vaginal route.43 PNG has excellent mucoadhesive properties and provides a constant release pattern, which is very useful for vaginal drug delivery. Tenofovir disoproxil fumarate (TDF) is an antiretroviral drug (a nucleotide analog) that works through the inhibition of viral reverse transcriptase. PNG of TDF was prepared with the help of cholesterol, surfactants (span 20, 40, 60, 80, tween 20 and 80), lecithin by coacervation phase separation method. A comparative in vivo dissolution study was conducted between proniosomes suppository, drug suppository and PNG formulations for 24 h using cellophane membrane, our results indicated the proniosomal suppository. Another result revealed a controlled and sustained release rate compared to the other two formulations.44 Terconazole, an antifungal drug, PNGs were developed on the basis of span 60 and brij 76 in different molar ratios (1:1, 1:1.5, and 1:2) relative to cholesterol. The results displayed that increased concentration of cholesterol relative to the surfactant affected both EE and vesicle size of niosomes prepared by incorporating into 1% carbopol gel. Drug release profiles from different prepared PNG formulations in simulated vaginal fluid studied in comparison with the commercial product of terconazole for 24 h. Depending on the high EE % and in vitro release profile, selected formulation was further evaluated for stability, mucoadhesion to the vaginal mucosa and inhibition of candida growth. Results indicated that the selected formula was in good stability and provided higher mucoadhesion and retention time than the commercial product, which resulted in more efficient in vitro inhibition of Candida albicans.45
Parenteral routes
In parenteral drug delivery, targeted and sustained drug release at a predetermined rate can be achieved due to remarkable advancement in pharmaceutical technology. Flurbiprofen46 and letrozole47 are prepared by the slurry method. Both drugs showed sustained activity and reduced dosing frequency.
Dermal and transdermal routes
The dermal route is employed for local action only to treat different types of skin disease. This route can avoid systemic effects and therefore offers fewer side effects. However, through transdermal delivery, we can deliver drugs for systemic action. But in both the dermal and transdermal drug delivery, the skin prevents the penetration of drugs. Vesicular drug delivery can be used to overcome this problem.
Non-steroidal anti-inflammatory drugs (NSAIDs) such as piroxicam,48 ketoprofen,49 meloxicam,50 celecoxib,51 and tenoxicam30 are planned to avoid gastrointestinal adverse effects. Here, all the NSAIDs except ketoprofen are prepared by the coacervation phase separation method, whereas ketoprofen is prepared by the slurry method.
Fluconazole-loaded PNGs were prepared by the coacervation phase separation method using different non-ionic surfactants (spans and tweens). The prepared fluconazole PNGs were evaluated for various parameters such as PS, drug EE %, and in vitro drug release. The experimental results showed that the EE % for the prepared formulae are acceptable (85.14-97.66%) and they are size (19.8-50.1 nm). The planned gel also showed sustained drug release. The formulation, which was prepared from span 60:tween 80 (1:1), and cholesterol showed highest EE % and gave slow release (40.50 ± 1.50% after 6 h), was subjected to ZP test, TEM as well as microbiological study. The results indicated a well-defined spherical vesicle with sharp boundaries and good physical stability of fluconazole within the prepared gel. Moreover, this formulation showed an excellent microbiological activity represented by a greater zone of inhibition (5.3 cm) compared with control gel (fluconazole in 2% hydroxy propyl methyl cellulose gel formula) (4.2 cm) and plain gel with no drug (0 cm) against C. albicans.52 Fang et al.53 studied transdermal estradiol gel and the results provided a higher permeation flux of estradiol across the skin. In vitro skin permeation study of dermal boswellic acid gel, was studied for 24 h, and a sustained release pattern was observed (84.83 ± 0.153 mg/cm2). Inhibition of inflammation of the proniosomal patch was also significantly (p<0.001) higher compared to the marketed gel at the same dose.54
In an attempt to modify the anti-hyperlipidemic effect and to reduce statins-induced hepatotoxicity, atorvastatin calcium (ATC) transdermal PNG was developed by a coacervation phase separation method. Different non-ionic surfactants (spans, tweens, cremophor RH 40, and brij 52) were incorporated in the vesicle’s lipid bilayer, along with lecithin. PNG gel was characterized for encapsulation EE %, vesicle size, polydispersity index (PDI), and ZP. The results revealed nano-sized (≤350 nm) range vesicles with relatively high ATC EE (70.12-88%). Ex vivo results of the selected formulation demonstrated the permeation superiority of ATC proniosomes over free drugs. The selected PNGs showed significantly high flux ranging from 4.23 to 8.46 µg/cm2 h - 1 with permeability coefficient values (P) (0.004-0.008 cm/h) when compared to free ATC dispersion, which significantly possessed lower flux and permeability coefficient results (2.92 µg/cm2h - 1 and 0.003 cm/h respectively). The pharmacodynamic study revealed that transdermal administration of ATC-PNG succeeded in retaining the antihyperlipidemic efficacy of orally administered ATC without elevating liver biomarkers. Histological examination signified the role of optimized ATC-PNG in hindering statin-induced hepatocellular damage.55 Transdermal cilostazole (CLZ) proniosomes were prepared by a coacervation phase separation technique. The optimum formula composed of 540 mg span 60 and 59.7 mg of cholesterol, had the highest EE % of (75.125 ± 0.125%), PS of (300.3 ± 0.2 nm), ZP of (-39.35 ± 0.15 mV), the percentage of the drug released after 2 h was (24.32 ± 0.13%) and after 24 h was (81.175 ± 0.325%). The safety of the proniosomes for topical application was confirmed by the histopathological examination. The CLZ-loaded proniosomes showed promising results with high potential to delivery it across the skin.56 CLZ loaded PNG was prepared by the coacervation phase separation method using span 60, cholesterol, and lecithin. The optimized formulation had the highest EE of 90% and an average PS of approximately 325 nm PDI reflected homogeneity in the formulation. ZP was -59.76 mV, high enough to indicate a stable formulation. The in vitro release studies manifested a sustained release behavior of clozapine from the PNG. The ex vivo permeation demonstrated noteworthy permeation of the drug through stratum corneum with a steady state flux of 18.26 ug/cm2/hr.57 Galantamine hydrobromide (HBr) is used for treating Alzheimer’s disease and is described as proniosome gel by coacervation phase separation method to overcome the side effects of oral delivery. Microscopical observations of the gels showed vesicles of optimum size from 3.030 - 3.735 mm. The gel also showed an optimum rate of spontaneity in the range 9.60 mm3 x 1000 to 11.80 mm3 x 1000 and EE of vesicles in the range 66.15% to 86.92%. The gels had pH in suitable range of skin (5.92-6.9). The in vitro drug diffusion studies revealed that the PNG containing tween 80 showed maximum drug diffusion (99.24%), whereas the gel containing span 20 showed minimum drug diffusion (71.74%).58
Intranasal routes
The nasal drug delivery method has some limitations like mucociliary clearance, degradation of drugs by the enzyme. Vesicular drug delivery systems can circumvent these limitations. Duloxetine (DX) is a new norepinephrine reuptake inhibitor used for treating depression. But it has high first-pass metabolism and low bioavailability (<50%) following oral administration, eventually leading to low cerebrospinal fluid concentrations. Khatoon et al.59 designed mucoadhesive thiolated chitosan (TCS) gel containing proniosomes of DX for intranasal drug delivery to enhance the drug’s contact time with nasal mucosa, bypass the first-pass effect and target the brain possibly using the olfactory pathway. Here, soya lecithin, cholesterol, and tween 80 was used in the preparation of the gel. pH of the DX-loaded proniosomal gel (D-MPNG) was 5.67 ± 0.145, indicating the compatibility of formulations within the nasal cavity without producing irritation. Notably, D-MPNG exhibited better control, releasing only 24% DX at pH 7.4 over 24 h compared to 78% release at pH 5.5. The presence of thiol groups of TCS significantly controls water uptake, resulting in moderate swelling and higher viscosity; thus, providing a sustained effect for a longer period.59
Cosmeceuticals application of proniosomes
Cosmeceuticals are generally used to refer to skincare products that contain active ingredients that are beneficial for improving the skin’s appearance and promoting healthy skin.60 Anti-aging cosmeceuticals are most frequently recommended by physicians, who use them as an integral part of a comprehensive skin rejuvenation program. Moisturizers and serum containing ingredients such as vitamin C, niacinamide, retinol, peptides, growth factors, and botanicals can all be used in this regard. Additionally, patients undergoing cosmetic procedures such as laser resurfacing and chemical peels may be given cosmeceuticals to prime the skin for procedures, encourage healing, and reduce complications after. Cosmeceuticals are also recommended for patients with acne, rosacea, eczema, and other skin conditions, where they are commonly used along with prescription medications. For example, moisturizers containing anti-inflammatory botanical ingredients may be used in conjunction with prescription medications for treating rosacea. Cosmeceuticals containing soy can be used to provide added skin lightening benefits when paired with hydroquinone.
Applying therapeutic and cosmetic agents onto or through skin requires a non-toxic, dermatologically acceptable carrier, which not only controls the release of the agent for prolonging action but also enhances the penetration to the skin layer.61 Proniosome gel meets such criteria, which are useful for the delivery of cosmetics and cosmeceuticals. The therapeutic agents which can be used for incorporation into proniosomal carrier systems include, moisturizing, nutritional, anti-wrinkle, anti-aging, cleansing, sunscreen particles, etc.
Proniosome is a potentially scalable method to produce niosomes for the delivery of hydrophobic or amphiphilic drugs.62 Anti-aging cream containing the methanolic purple glutinous rice extract loaded in niosomes was developed by Manosroi et al.63 Anthocyanin present in purple glutinous rice extract is responsible for the anti-aging activity. After 6 cycles of heating and cooling test, the formulation with 1% w/v of the purple glutinous rice extract contained 52.28% anthocyanin of the initial. For in vivo antiaging activities, a cream containing niosomes loaded with the extract gave significantly decreased melanin index and skin roughness reduction of 14.05 and 9.95% of the initial, respectively. The percentage changes of the increased skin hydration, skin elastic extension, and skin elastic recovery when applied on human volunteers’ skin with this formulation were +48.73, 24.51, and +35.98%, respectively.
Tretinoin (TRT) is a widely used retinoid for the topical treatment of acne, photo-aged skin, psoriasis, and skin cancer. TRT-loaded proniosomes were prepared by the slurry method with the help of span 60 and D-sorbitol, span 40, cholesterol 95% stabilized, and tween 20.64 prepared hydrated proniosomes were characterized by an evaluation of PS, the effect of drug concentration, EE, etc. EE of all hydrated proniosomal dispersions ranged from 76.6 ± 0.001% prepared to use span 40 to 94.15 ± 0.041% prepared using span 60. In vitro drug release was increased till 5th hour.
Diferuloylmethane or curcumin is obtained from turmeric, which possesses infiammatory properties blocking the formation of reactive oxygen species.65 Proniosomes of curcumin were prepared using non-ionic surfactants (tween 80, span 60) either solely or in combination with cholesterol. The highest encapsulation efficiency of curcumin in niosomal formulations was 99.74%. Kinetically, niosomes fitted to the Korsmeyer-Peppas model with non-Fickian transport. The anti-inflammatory activity of curcumin in various formulations was evaluated using a rat hind paw edema method and the percentage of swelling was 17.5% following 24 h in the group treated with curcumin niosomal emulgel.66
Coenzyme Q10 (CoQ10) also known as ubiquinone, an essential compound is found in every cell of the human body. CoQ10, an essential compound of cellular bioenergetics, also acts as a strong antioxidant and protects the body against aging.67 CoQ10 proniosomes were prepared using the standard method with the help of Q10, span 85, soya lecithin, and cholesterol. In vitro drug release of CoQ10 followed a special cubic model, as the statistics of the chosen model were found significant (p= 0.0006). The change in the levels of soy lecithin had a great impact and showed a synergistic effect on the release characteristics. A skin permeation study showed that the cumulative amount of CoQ10 permeated in 12 h was found to be 515.85, 463.25, and 507.49 mg/cm2, respectively for selected PNG formulations. Animal skin was treated with UV radiation followed by treatment of PNG CoQ10 and conventional CoQ10 present in a gel base. The effectiveness of the treatment was evaluated based on biochemical estimation and histopathological studies. By using CoQ10 PNG formulation, levels of superoxide dismutase, catalase, glutathione, and total proteins were restored by 81.3%, 72.1%, 74.8%, and 77.1%, respectively to that of the control group. Histopathological studies revealed better protection of skin treated with CoQ10 PNG compared to free CoQ10. The prepared PNG did not interact with the normal histology and, hence, tolerated by the animal skin compared to conventional gel. Assessments of the formulations for various enzymatic and non-enzymatic estimations in animal skin after UV irradiation proved the efficacy of the developed formulation.
Proniosomal formulations containing the natural antioxidant resveratrol (trans-3,5,4¢-trihydroxystilbene, RSV) were prepared by Schlich et al.68 RSV is a polyphenol compound having anti-inflammatory,69 neuroprotective,70 anti-aging,71 and anticancer effects.72 Proniosomal powders were prepared by the slurry method and characterized. The hydration and sonication of proniosomes resulted in the formation of lipid nanoparticles with a mean diameter in the range 180-300 nm and a highly negative surface charge. RSV release from proniosome-derived niosomes was investigated in simulated gastric and intestinal fluid. biocompatibility assay carried out on intestinal cells (Caco2) demonstrated that proniosomes prepared with an HLB of 13.5 were significantly less toxic than their HLB16.7 counterpart. All the tested formulations could be employed safely at the doses commonly administered by the oral route.
Canthaxanthin (CTX) is a xanthophyll (a subclass of carotenoids) with widespread applications in pharmaceutical and cosmetic industries. It is a superior antioxidant and scavenger of free radicals compared with carotenoids such as b-carotene.73 CTX was encapsulated in proniosome powders, which were were prepared with an equimolar ratio of span 60/cholesterol and four different carriers, namely, maltodextrin, mannitol, lactose, and pullulan.74 The study showed that the niosomes produced by hydration and sonication of the proniosomes were small (≤200 nm) and quite homogeneously dispersed (PDI ≤0.3). The encapsulation efficiency of CTX in formulations varied between 55.3 ± 1.8% and 74.1 ± 2.7% after hydration and sonication. Although light and high temperatures affected the stability of CTX drastically, encapsulation in proniosomes retarded its degradation. This formulation can provide convenient, non-toxic, and inexpensive vehicles for dissolving and stabilizing CTX in functional food products.
O-padimate is a UV-B filter widely used as a sunscreen agent. A study investigated the combined influence of 3 independent variables in the preparation of O-padimate proniosomes, which were prepared by the slurry method with span: Brij, surfactant. The developed gels were characterized for vesicle size, morphology and EE, skin permeation studies, rheological properties, and sun protection factor (SPF). Results reveal that optimized O-padimate proniosomal formulations showed high SPF and low transepidermal water loss.75
Rutin (Rut) is a natural flavonol that has various therapeutic properties including antioxidant and antitumor activities. Rut PNG for cutaneous applications was designed to improve the poor aqueous solubility of Rut. The gel was prepared by the coacervation phase-separation method and complies with the standard requirements in terms of PS (140.5 ± 2.56 nm), ZP (-27.33 ± 0.09 mV), encapsulation capacity (>50%), pH (7.002 ± 0.18), and rheological properties. The results showed high biocompatibility of the gel on the 3D reconstructed human epidermis model characterized by increased viability of the cells and lack of irritant and phototoxic potential. The values on 2D cells confirmed the preferential cytotoxic effect of Rut on melanoma cells (IC50 value: 8.601 µM, nuclear fragmentation) compared with normal keratinocytes. Our data suggest that the PNG is a promising drug carrier for Rut in the management and prevention of skin disorders.76
Clinical trials with proniosomes
Selected proniosomal formulation of VP was tested in vivo to compare the pharmacokinetics of VP from a proniosomal patch containing VP (treatment A) to an oral commercial tablet containing the same dose of VP (treatment B) using a non-blind, two treatment, two-period, randomized, crossover design.77 Twelve healthy non-smoking male volunteers (26-37 years, 78-96 kg) participated in the study and were randomly assigned to one of the two treatment groups of equal size. The study results showed significant differences in the shape of the concentration-time courses between the two treatments. For VP oral tablet a rapid sharp peak was found at 1.5 h followed by a fast decline in plasma drug levels. However, for the VP proniosomal patch, the absorption was much slower and extended over a longer period. Moreover, the patch exhibited higher drug levels in the plasma from 6 to 12 h compared to the tablet. The average Cmax was significantly lower (p<0.05) for the patch (12.44 ± 1.87 ng/mL) compared with the oral tablet (63.69 ± 8.32 ng/mL), while tmax was significantly higher (p<0.05) in the case of the transdermal patch (12 h) compared with the oral tablet (1.5 h). The extent of absorption of VP from the patch expressed by AUC0-t was determined to be about 101% larger and statistically significantly different compared to the oral tablet. The relative bioavailability of VP proniosomal patch to the oral tablet was estimated to be on average 206%. The elimination half-lives of VP after oral and transdermal administration were 1.36 ± 0.27 h and 13.94 ± 1.2 h, respectively, and were statistically significant (p<0.01).77
TRT-loaded proniosomes were prepared by the slurry method with the help of span 60 and D-sorbitol, span 40, cholesterol 95% stabilized, and tween 20. The planned PNG was studied clinically on 12 Egyptian patients aged more than 18 years [2 males and 10 females; with an average of 20 (± 4 years) with acne (papules, closed comedones, and open comedones)] on their face.64 The result showed only very slight erythema (score: 0.143 ± 0.377) for TRT PNG compared to 0.025% TRT gel (score: 1.70 ± 0.755). Similarly, the marketed product displayed an erythema score of 1.50 ± 0.534 and could not diminish the irritation caused by topical application of TRT. The overall improvement of the individual lesions was also better than the marketed product during the 4 week study period.
Niosomal (hydrated form of proniosome) benzoyl peroxide (BPO) and clindamycin (CL) lotion was prepared and compared with niosomal CL in acne vulgaris. In both cases, the concentration of the drug was 1%. A double-blind clinical trial study on 100 patients with acne vulgaris was conducted in Afzalipour Hospital in Kerman (Iran).72 The efficacy of treatment protocols was evaluated in the 2nd, 4th, 8th, and 12th weeks of treatment by counting lesions (severity and grading acne lesions) and quality of life. Furthermore, the side effects were evaluated at each treatment visits. The reduction in the mean percentage of acne lesions in the case group (treated with BPO 1% and CL 1%) (64.21%) was higher than that the control group (treated with niosomal CL 1%) (59.04%), but the statistical difference was not significant. A sum of excellent and good results was found in 80% and 76.1% of the case and control groups, respectively (p= 0.377). Also, adding BPO to the treatment formulation in the case group did not increase adverse effects, as the statistical difference between the 2 groups was not significant (Table 3).78
CONCLUSION
Proniosomes could be a promising vesicular drug delivery method for the future. They are one of the drug carriers in vesicular drug delivery methods, which is a better alternative to liposomal drug delivery due to its controlled and sustained action and provide better physical, chemical stability, and potentially scalable for commercial viability. They offer excellent potential for improved drug delivery through versatile routes such as oral, parenteral, dermal, transdermal, ocular, vaginal, pulmonary, and nasal by overcoming the permeation barriers faced by several drugs. Different types of unit dosages form like tablets, capsules, and beads can be prepared with the dry form of proniosomes. Due to the versatility of proniosomes, they are widely investigated as drug carriers. There is a lot of scope to investigate new carrier materials for the preparation of proniosomes and their potential remains to be investigated to the full extent.
Ethics
Peer-review: Externally and internally peer-reviewed.
Authorship Contributions
Concept: M.A., Design: M.A., F.A., Data Collection or Processing: F.A., Analysis or Interpretation: M.A., F.A., Literature Search: M.A., F.A., Writing: M.A., F.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.