INTRODUCTION
Various novel technologies and approaches are being explored for medicinal compounds to be developed as modified release dosage forms. These forms provide significant benefits compared with the immediate release (IR) dosage forms.1,2 These include less frequency of administration, safer, and effective plasma concentrations and better patient compliance. Many investigations on the design of modified release dosage forms aim to develop single unit forms such as matrix tablets. Fewer studies are conducted on the development of multi-unit forms such as microcapsules or pellets.3,4 The multi-unit forms have beneficial characteristics such as well-controlled drug release, no burst release of drug and are evenly dispersed in the gastrointestinal tract after oral administration.5,6 There are some reports on the design of pellets for sustained release (SR).7,8 While developing a SR dosage form, the approach employed should take into consideration the physicochemical properties of the drug, especially the solubility. This is because controlling the release of highly soluble drugs is difficult because drug release occurs by diffusion form the unit and higher the solubility, more will be the diffusion of the drug. It is essential that the formulation and echnology are appropriately developed.
Extrusion and spheronization is a widely used technique for preparing pellets with different characteristics, including obtaining the SR of drugs. Mahrous et al.7 have developed SR pellets of indomethacin by different concentrations of hydrophilic polymers, polyethylene glycol 4000, hydroxypropyl methylcellulose E5 LV premium (HPMC) and polyvinylpyrrolidone K30. SR matrix pellets of flurbiprofen were developed and drug release optimized by studying the matrix forming units employing HPMC and carbopol.8 Sriamornsak et al.9 have prepared alginate pellets of theophylline employing microcrystalline cellulose (MCC). They studied the drug-release properties of the pellets by varying the number of other substances in the formulation, such as sodium alginate and calcium salts. Chatchawalsaisin et al.10 developed MCC pellets of acetaminophen and diclofenac sodium. They explored a range of levels of glyceryl monostearate to evaluate its effect on spheronization and drug release.
In all these various earlier investigations, MCC is employed as the spheronizing agent to form the pellets. However, of late there has been an increasing interest shown by researchers to develop alternative substances to MCC as spheronizing agents. This is because of adsorption of some drugs to MCC and undesirable chemical interactions between drugs and MCC.11,12 Several investigators have worked on polymers such as carrageenan, chitosan and starch - dextrin etc. to prepare pellets by extrusion and spheronization.13,14,15
For obtaining the SR from the pellets of a highly water-soluble drug, it is essential that the matrix of the pellet does not allow for rapid diffusion of the soluble drug. In this regard, investigators in the past have explored different hydrophilic polymers along with MCC. However, there are only a few studies have been undertaken in which waxy/fatty or lipoidal materials are explored for preparing the pellets.16,17
In our present investigation, we studied the role of gelucire (43/01), a fatty polymer, in developing SR matrix pellets by extrusion and spheronization using almond gum as the alternative spheronizing agent to MCC. The gelucires with a low hydrophilic-lipophilic balance value are hydrophobic and are suitable for designing SR products.18 Almond gum is an exudate obtained from the plant Amygdalus communis L.19 There are some reports of almond gum as a binder in tablet formulations and for obtaining SR.20 Almond gum nanofibers were investigated and reported to help obtain SR.21
In our laboratories, we developed SR matrix tablets of employing almond gum and pellets employing almond gum as a substitute for MCC in the extrusion and spheronization process.22,23 Therefore, this investigation explores the usefulness of the combination of hydrophobic gelucire and almond gum in preparing SR pellets. Diltiazem hydrochloride was selected as a model, highly water-soluble drug. It is used for treating angina and in the management of hypertension.24
MATERIALS AND METHODS
Materials
Diltiazem hydrochloride (gift sample from Julphar Gulf Pharmaceutical Industries (UAE), gelucire (43/01) was a gift sample from Gattefosse (Paris, France). Almond gum was procured from Harekrishna Herbals (India). All other chemicals and solvents were of analytical grade.
Preparation of pellets by extrusion and spheronization
The required quantities of diltiazem hydrochloride were incorporated into gelucire (43/01) which was melted at 50°C. After cooling and solidification, the mixture was ground and sifted through a 250 µm sieve. This powder mixture was employed during the pelletization process rather than the separate quantities of drug and gelucire. The powder mixture was mixed with appropriate quantities of almond gum and wetted with water until a homogenous and cohesive mass was obtained. The mass was then fed manually into an extruder (Shakti model EX-50/SSP120) fitted with a die/screen of 1 mm diameter operated at a speed of 30 rpm. The extruded mass was spheronized in a unit fitted with a crosshatched plate. A spheronization speed of 1000 rpm was employed and the spheronizer was operated for 15 min. The resulting pellets were air dried at room temperature for further use.
To study the effect of variables, pellets were prepared using 32 factorial design (Table 1). Amounts of gelucire and almond gum were selected as the two independent variables. The influence of the independent variables on the size, friability of pellets and drug release was studied.
Characterization of the pellets
Yield and drug content
Pellets were weighed after drying and percent yield was calculated employing the formula given below.
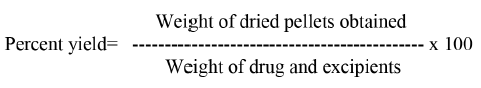
For determination of drug content, 10 mg pellets were triturated and the drug diltiazem hydrochloride was extracted into distilled water by sonication for 30 min. The solution was filtered and analyzed spectrophotometrically at 240 nm (Shimadzu model UV 1600) after sufficient dilution with water.
Friability, Carr’s index, and angle of repose
Friability
For each study, 25 g pellets were mixed with 20 glass beads (3 mm in diameter) and were placed in Roche friabilator and operated for 8 min at 25 rpm. After the study, the pellets were sieved through a sieve of 250 µm opening and the weight of pellets left on the sieve was determined and the friability was calculated using the formula given below.
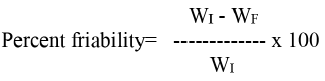
WI and WF are the initial and final weights of the pellets.
Carr’s index
Pellets (100 g) were added to a graduated cylinder and density apparatus (VTAP MATIC II). The bulk and tapped densities were determined. From the obtained values, the Carr’s index and Hausner ratio were calculated employing the formulae below.
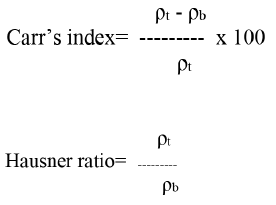
ρt is the tapped density and ρb is the bulk density.
Angle of repose
The angle of repose was measured according to the fixed funnel method. The funnel is positioned in a way that the lower tip of the funnel is at a fixed height of 3 cm from a horizontal surface. The pellets were then passed slowly through the funnel in such a way that the upper tip of the surface of the heap being formed just touched the lower tip of the funnel. The angle of repose is determined from the height and radius of the heap formed.
Average pellet size
The average size of pellets of different formulations was estimated by sieve analysis (electrolab model EMS 8). 100 g pellets were sieved employing a set of sieves. The pore size of different sieves was between 150 and 2000 microns. After shaking the sieve set for 10 min, the weight of pellets on each sieve was determined to determine the size of pellets.
Surface and shape characterization of pellets
The surface and shape of pellets were studied by optical microscopic image observation and scanning electron microscopy (SEM).
Optical microscopy
The pellets were observed employing an Olympus microscope (model BX 53) attached with DP 74 digital camera. The images were taken employing an image analyzer connected to a black and white camera and processed using cellSens software.
SEM
Pellets were placed on carbon tabs (agar scientific) and imaged under low vacuum on a JEOL JSM5310LV SEM at an accelerating voltage of 20 kV. The aspect ratio of the particles was measured by appropriately thresholding the images using AZtec version 3.3 software (Oxford instruments).
Differential scanning calorimetry (DSC)
DSC studies were performed to know about any interaction between diltiazem hydrochloride and the two excipients used in the preparation of pellets. The calorimeter (Shimadzu DSC 60+) was run at a scanning speed of 10°C/minute. The temperature range of heating was 25-250°C. After sealing the samples in aluminum pans, heating was carried out in an inert atmosphere which is maintained by circulating nitrogen gas.
Fourier-transform infrared (FTIR) spectroscopy
The infrared spectroscopic analysis of diltiazem hydrochloride and the mixtures of diltiazem with almond gum and gelucire was performed by attenuated total reflectance sampling interface technique using Agilent model Cary 630.
Drug release study
Release of diltiazem hydrochloride from different pellets was studied by employing USP dissolution rate test apparatus type I. 0.1 N hydrochloric acid for first 2 h and phosphate buffer of pH 7.4 for the remaining 10 h were used as medium for the release study. Pellets containing 90 mg of diltiazem hydrochloride were added to the drug release medium. The various removed sample media at different time points were assayed for diltiazem hydrochloride released by determining the absorbance at 240 nm.
Statistical analysis
Responses obtained from factorial design experiments were subjected to multiple regression analysis using SPSS statistics 24 software. Data obtained on the 3 dependent variables, size of pellets, friability and drug release for all formulations (F1-F9) showed a wide variation, which indicated that the response values of dependent variables highly rely on the independent variables.
RESULTS AND DISCUSSION
In this investigation, pellets were prepared by extrusion and spheronization without the conventional and widely used spheronizing agent, MCC. Almond gum is investigated as a spheronizing agent and its usefulness in acting as a release retardant matrix material is studied. We previously reported on the SR pellets of furosemide prepared by extrusion and spheronization employing almond gum.23 Our preliminary investigations on the suitability of almond gum alone for preparing SR pellets of a highly water soluble drug such as diltiazem hydrochloride revealed that although SR could be obtained, the release could not be significantly retarded. So we employed a combination of lipophilic (gelucire 43/01) and hydrophilic (almond gum) materials as release retardants. A 32 factorial design (Table 1) was used to determine the effect of the two variables (percentage of almond gum and gelucire) on the three dependent variables, size, and friability of pellets and drug release.
Characterization of pellets
Preliminary characterization
The yield of the pellets prepared in different formulations was between 86 and 92%. The changes in almond gum or the gelucire proportions did not majorly influence the percentage yield. The drug content of various pellets was found to be between 90 and 94%. The results of the study of flow properties are shown in Table 2. From the angle of repose values, it can be concluded that the flow properties were good for the pellets, especially the formulations F1 to F3. An angle of repose value below 25° suggests a good flow. An angle of repose lower than 40° indicates good flowability, conversely an angle of repose superior to 40° is an indication of cohesiveness.25,26 However, it is observed that as the proportion of gelucire increased, the flow became poorer, as reflected in the various values shown in Table 2. The fatty nature of gelucire probably impedes the flow of the pellets. Also, it is observed that for a given proportion of gelucire, with a higher amount of almond gum, the flow appears to be improving. It was probably because the pellets were becoming more regular and rounded off with higher amounts of almond gum (discussed more in detail below).
The friability of different formulations of pellets was found to be between 0.52 and 3.68%. Pellet formulations, in which the fatty substance gelucire proportion, was higher exhibited higher friability. Pellet friability is an index of pellet strength, with a lower friability reflecting greater pellet strength. Increased amounts of gelucire probably resulted in a decrease in the amount of water available during extrusion stage, resulting in poor quality extrudates which could not be spheronized into pellets of good strength. Reynolds27 reported their work that by increasing the amount of water content in the extruded mass, the friability problem can be overcome. As the water content increases, the association of water molecules with the binder almond gum will be greater, which will result in higher plasticity and greater binding capacity. As the gelucire proportion increases, it probably has a more impeding effect on the availability of water in the extruded mass, resulting in less compact extrudates and more friable pellets.
Size of the pellets
The sizes of pellets of different formulations are shown in Table 2. The size of the pellets varied as the amounts of gelucire and almond gum in different formulations changed. With increase in the amount of gelucire, the size of the pellets decreased. However, for a given amount of gelucire present, an increase in almond gum-yielded pellets of higher size. So, gelucire and almond gum had negative and positive influences on the size of the pellets, respectively. Uniform wetting of the dough mass is essential for good-quality extrudates to be formed. The presence of gelucire probably hindered the movement of water during extrusion process and the extruded mass had lower plasticity, which is essential during spheronization. Pellets formed from extrudates of higher amounts of gelucire did not have sufficient strength and were broken into smaller pellets during spheronization. As the almond gum concentration increases, the binding strength and the plasticity of the extrudates increases. A similar observation of increase in pellet size with higher binder amount was reported by other investigators.28,29
The proportion of the two ingredients, e.g. gelucire and almond gum (the two independent variables), also affected the size distribution of the pellets, as shown in Figure 1. With increase in the amount of gelucire, not only the average pellet size decreased but also affected the size distribution of the pellets in different formulations. As the amount of gelucire increased, it resulted in a size distribution, which was more. For example; between F1 and F4 formulations, with higher gelucire in F4 and with almond gum being the same in both, pellets of F4 formulation exhibited a non-uniform distribution compared to F1. A similar observation can be made in F2 and F7 formulations, with pellets of F7 formulation showing non-uniformity in distribution.
Surface and shape characterization of pellets
The surface features of pellets were investigated by optical microscopy and SEM. The pellets were found to be spherical in shape and exhibited a smooth surface (Figure 2). However, higher amount of gelucire in the pellet composition (F9 pellets) resulted in pellets, whose surfaces were not smooth and showed irregularity as shown in Figure 2A, whereas with lesser amounts of gelucire and proportionately higher amounts of almond gum (F3 pellets), yielded pellets that are more spherical and have smoother surface as shown in Figure 2B. The SEM photographs are shown in Figure 3 that confirm the spherical and more regular features of the pellets with higher almond gum (Figure 3B) and a surface irregularity with pellets of higher gelucire (Figure 3B).
An important feature of a pellet is its roundness. Aspect ratios of the pellets were used to express their roundness. Podczeck and Newton30 have carried out image analysis to evaluate the shape of pellets and suggested an upper value for the aspect ratio of 1.1 for a pellet to be described as spherical. The pellet shape and spherical nature are critical in ensuring the uniform filling of pellets into a hard gelatin capsule. Rowe et al.31 have reported that an aspect ratio of less than 1.2 will ensure reproducibility of the filling process. Since the pellets made in this investigation needed to be developed into hard gelatin capsules, we determined the aspect ratio of various pellets. The aspect ratio (shown in Table 2) was found to be between 1.01 and 1.17. These values suggest that the pellets prepared are spherical and can be handled easily in the subsequent stages of processing.
DSC
The DSC thermograms of diltiazem hydrochloride and the mixtures of diltiazem with gelucire and almond gum are shown in Figure 4. Diltiazem showed a peak at 212°C, which is due to the melting of the drug.32 The melting peak of diltiazem was retained at the same temperature even in the mixtures with gelucire and almond gum indicating absence of any interaction between diltiazem and the two excipients used in the pellet preparation.
FTIR spectroscopy
The FTIR spectra of diltiazem hydrochloride and its mixtures with gelucire and almond gum are shown in Figure 5. Diltiazem hydrochloride exhibits strong to medium characteristic absorption peaks at 1743 cm-1 (acetate C=O stretch), 1679 cm-1 (lactam C=O stretch), 838 cm-1 (O-substituted aromatic C-H out-of-plane deformation) and 781 cm-1 (p-substituted aromatic C-H out-of-plane deformation).32 These principal peaks of diltiazem were retained in the mixtures of the drug with gelucire and almond gum, suggesting that there is no chemical interaction with the two excipients used.
Drug release
The drug release from the pellets prepared was found to be slow and sustained (Figure 6). But the release rate is different in various pellet formulations depending on the percentage of almond gum or gelucire employed. Almond gum and gelucire were employed at 3 different levels. As their proportion of increased, drug release rate decreased. A 2 factor 3 level factorial design was employed to study the influence of these two variables. In F1 and F2 formulations, though the release was slow, all the drug was completely released by the end of 6th hour. In F3 and F4, the release is slightly more retarded but completed by 8th hour. An interesting observation is that in F3 and F4 pellets, the release was almost the same, even though the almond gum was 50% less in F4. This is probably because gelucire’s percentage in F4 is 50% more than in F3. So, this higher gelucire compensates for the less almond gum in controlling the release. But in pellets of F5 and F6 formulations with the same gelucire amount as F4, the release is slower, this is because the almond gum is higher. This confirms that, while gelucire contributes to the retarding effect of the drug, increased amounts of almond gum play an important role in retarding the release.
The theoretical desired release rate required from diltiazem hydrochloride is calculated from its pharmacokinetic parameters.32 The biological half-life of diltiazem is 3.5 h. The normal conventional IR dose of diltiazem is 30 mg. The following equations were used to calculate the SR dose and the desired release rate for 12 h.
DI= Immediate release normal dose= 30 mg
t1/2= Biological half-life= 3.5 h
KE= Elimination rate constant= 0.693/3.5 = 0.198 hr 1
K0= Desired release rate for sustained dose= DI x KE= 30 x 0.198= 5.94 mg hr-1
DI= K0/KE= DS/KE x T (because K0= DS/T)
Therefore, DS= DI x KE x T= 30 x 0.198 x 12= 71.28 mg
DI*= Corrected initial dose= DI - (tp x DI x KE),
where tp is the time for the peak plasma concentration
So corrected initial dose= 30 - (2 x 30 x 0.198)= 30 - 11.88= 18.12 mg
Total dose= DI + DS= 18.12 + 71.28= 89.4 ~ 90 mg
The desired release rate= 5.94 mg hr-1
Of the various pellets, F6 formulation exhibited a release profile close to the theoretical desired release. In products F7 to F9, the release becomes much slower and is incomplete because of higher amounts of gelucire and almond gum. In spite of almond gum quantity being higher in F9 than in F8, the release profiles are almost the same in F8 and F9. This is probably because gelucire at higher levels (being same in F8 and F9) has a significant retarding effect on the release. Thus, it is evident from the release profiles that with the changes in the proportion of gelucire and almond gum in the pellets, the release rates varied. This observation is further confirmed in the statistical analysis that is conducted on the data obtained (discussed below).
To know the drug-release mechanism, the drug release data are analyzed as per first-order, Higuchi33 and Korsmeyer et al.34 models. The r2 values in various models are given in Table 3. The various values of release rate constants and the “n” values of Peppas plot suggest that the drug release from the pellets is by non-Fickian anomalous diffusion.
Data analysis of percent release in 6 h
The regression equation that predicts the percentages released is given below:
Y (percent released)= 150.611 - 2.7 X1 - 0.817 X2
The observed value for percent release for all 9 formulations (F1-F9) varied from 39.45% to 100% among the formulations. Correlation coefficient value was found to be 0.949 and can be considered a good measure of the quality of the prediction of the dependent variable using the model employed. Negative signs of coefficients of X1 and X2 in regression equation indicated retarding effect of independent variables on the response (drug release). The higher coefficient value of X1 (gelucire) suggests the more retarding effect shown by gelucire on drug release than the other variable almond gum. This substantiates the earlier discussion made on the drug release.
Data analysis of size of pellets
The regression equation that predicts the size of pellets is given below:
Y (size of pellets)= 1106.667 - 24.1 X1 + 26.833 X2
The observed values of size of pellets ranged from 1146 to 1458 µ. A negative sign on the coefficient for X1 and a positive sign on the coefficient X2 indicates that gelucire has an effect that reduces the size of the pellets, whereas almond gum has the opposite effect to resulting in pellets of higher size. A correlation coefficient value of 0.985 indicates a good level of prediction.
Data analysis in friability of pellets
The regression equation that predicts the friability of pellets is given below:
Y (percent friability)= 0.9116 + 0.114167 X1 - 0.04055 X2
A positive sign on the coefficient for X1 and a negative sign on the coefficient of X2 confirm the earlier discussion that higher amounts of gelucire in the pellet formula increase the friability, while almond gum reduces the friability.
The adequacy of the fitted model was verified by ANOVA and results are shown in Table 4. The various values from ANOVA tests indicate that the independent variables statistically significantly predict the dependent variables.
CONCLUSION
SR pellets of diltiazem hydrochloride could be prepared by extrusion and spheronization without the inclusion of MCC in pellet manufacture and by using almond gum as the extrusion and spheronization agent and matrix former for the SR. Dispersion of the drug in gelucire before it was converted to pellets resulted in extended release of the drug. A 32 factorial study and the multiple regression analysis helped investigate the effect of two independent variables, almond gum and gelucire, on the characteristics of the prepared pellets and the drug release. The flow properties, size, and friability of the pellets are affected by the proportions of almond gum and gelucire. The drug release rate changed with the changes in the proportion of the pellet composition. The results of the study suggest that employing gelucire (43/01) in the preparation of pellets is a useful approach in the design of SR products of highly water-soluble drug such as diltiazem hydrochloride.
ACKNOWLEDGMENTS
The authors express their gratitude to the President of RAK Medical and Health Sciences University (UAE) and Dean of RAK College of Pharmaceutical Sciences for their encouragement and support in conducting the research.
Ethics
Ethics Committee Approval: The study was approved by the RAK Medical and Health Sciences University Ethics Committee (approval no: RAKMHSU-REC-021-202/21-F-P).
Informed Consent: There was no investigation carried out using human volunteers.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Concept: R.V.K., Design: R.V.K., O.S., H.Y., J.B., Data Collection or Processing: R.V.K., O.S., H.Y., J.B., Q.I., Analysis or Interpretation: R.V.K., O.S., H.Y., J.B., Literature Search: R.V.K., O.S., H.Y., J.B., Writing: R.V.K., O.S., H.Y.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.